The pharma intermediates market is at a technological crossroad, accelerated by innovation, automation, and ever-increasing regulatory demands. As a core building block of modern chemical and pharmaceutical manufacturing, intermediates like Refine N,N'-Dimethyl Urea are the engines powering drug synthesis, polymer modification, and specialty chemistry worldwide. This analysis explores detailed technological parameters, manufacturing flow, supplier comparison, real-world case studies, and authoritative industry references for pharma intermediates, with a focus on leading additives like dimethylurea and specialty additives for polymers.
1. Industry Trends in Pharma Intermediates: 2024 Global Outlook
- Market size (2023): $40+ billion globally; CAGR (2024-2029): 6.1% [Grand View Research]
- Key Growth Drivers: Biopharmaceutical innovation, advanced drug synthesis, green & sustainable chemistry
- Dominant application sectors: Pharmaceuticals, Agrochemicals, Polymers, Electronics
- Notable Intermediates: N,N′-Dimethyl Urea, Acetophenone derivatives, Tetrahydrofuran compounds
- Regulatory landscape: Strict global compliance with ISO 9001:2015, cGMP, FDA/EMA guidelines
2024 Parameter Comparison for Common Pharma Intermediates
| Name |
Chemical Formula |
Purity (%) |
Melting Point (°C) |
Main Applications |
ISO Grade |
| N,N′-Dimethyl Urea |
C3H8N2O |
≥99.5 |
105-109 |
API Synthesis,
Polymer Additives |
ISO 9001:2015 |
| 4-Aminophenol |
C6H7NO |
≥99.0 |
187-190 |
Paracetamol, Dyes |
ISO 14001 |
| Tetrahydrofuran |
C4H8O |
≥99.0 |
-108.4 |
Polymerization, Solvents |
cGMP |
| Dimethyl Sulfate |
C2H6O4S |
≥98.5 |
18 |
Alkylating agent |
ISO 45001 |
2. Technical Specifications of Refine N,N'-Dimethyl Urea
Key Specifications
- Product Name: Refine N,N'-Dimethyl Urea
- Chemical Formula: C3H8N2O
- CAS No: 96-31-1
- Molecular Weight: 88.11 g/mol
- Appearance: White crystalline powder
- Purity: ≥99.5% (HPLC)
- Melting Point: 105~109°C
- Moisture: ≤0.2%
- Chloride (Cl): ≤0.005%
- Sulfate (SO4): ≤0.005%
- Heavy Metals: ≤10 ppm
- Packaging: 25kg/drum or custom
- Standard: ISO 9001:2015 / ANSI / GOST R
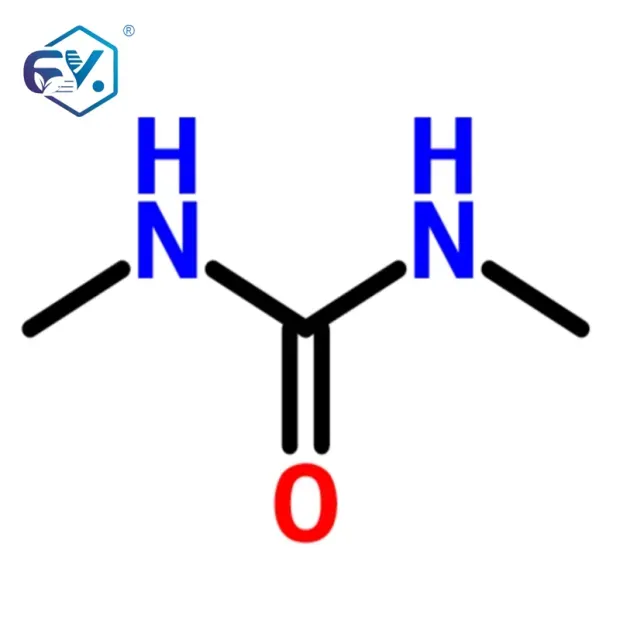
High Purity Dimethylurea for Polymer & Pharma Applications
Technical Parameters vs Industry Average
Graph: Side-by-side technical data comparison for pharma intermediates (source: Manufacturer QC/ISO testing, 2024)
3. The Refine N,N'-Dimethyl Urea Manufacturing Process Explained
Flow Diagram – Industrial Synthesis of Dimethylurea

- Raw Materials Dosing: Methylamine and urea (analytical grades) are precisely fed into a stirred reactor.
- Catalytic Reaction (60-75°C, pH 6.5): Synthesis proceeds under tightly regulated temperature & pH, monitored using PID controllers. Side reactions minimized for high selectivity.
- Crystallization & Filtration: Under slow cooling, dimethylurea precipitates as a white crystal; filtered and washed in deionized water.
- Drying (Vacuum Oven, 60°C): Achieves ultra-low residual moisture (<0.2%).
- Sieving & Packing: Final inspection (XRD/GC) and nitrogen-packed to ensure stability.
- QC & Certification: Product analyzed for ISO 9001:2015 and meets ANSI/ASTM E29 standards.
Each node strictly follows FDA/EMA/ISO GMP protocols — ensuring safety & reproducibility in pharma intermediates supply.
4. Engineering Material Choices & Manufacturing Processes
Refine N,N'-Dimethyl Urea leverages high-grade raw materials, guaranteeing minimal heavy metal, chloride, and sulfates impurities. Production integrates advanced CNC-controlled reactors for precision and includes:
- Chemical Synthesis: Automated PLC/PID batch reactors, for reproducible quality
- Crystallization: Controlled-rate cooling to maximize yield and consistency
- Packing & Stability: Nitrogen atmosphere, oxygen/moisture barriers extending shelf life to 36 months
- Inspection: HPLC, GC-MS, XRF for impurity detection. Batch CoA issued per EN10204 3.1 industry certification
- Compliance: ISO 9001, ANSI/ASTM standards, and FDA/EMA cGMP (when applied to pharmaceutical use)
Why does this matter?
Material consistency and manufacturing precision are essential for pharma intermediates that enter APIs (Active Pharmaceutical Ingredients) or critical additives for polymers.
5. Product Data Visualization – Refine N,N'-Dimethyl Urea vs. Competitors
Product Comparison Table: Refine N,N'-Dimethyl Urea vs. Industry Peers
| Brand/Product |
Purity (%) |
Moisture (%) |
Heavy Metals (ppm) |
ISO Standard |
Shelf Life (Months) |
Traceability |
| Refine N,N'-Dimethyl Urea |
≥99.5 |
≤0.2 |
≤10 |
ISO 9001:2015, ANSI |
36 |
Full, Batch+QR |
| Peer A |
98.8 |
0.3 |
22 |
ISO 9001:2015 |
24 |
Limited |
| Peer B |
98.2 |
0.4 |
30 |
cGMP |
18 |
Partial |
Chart: Purity (%) comparison among pharma intermediates
Refine N,N'-Dimethyl Urea Usage in Application Sectors
Pie: Top 3 fields—pharma, polymers, agrochemicals—account for 87% of usage
6. Application Scenarios and Technical Advantages
- Active Pharmaceutical Ingredient (API) Synthesis: Used in producing antibiotics, antidiabetics, antihypertensives due to high purity and process yield
- Additives for Polymers: Increases thermal stability, UV-resistance, anti-aging properties in polyamides, polyurethanes, and engineering plastics
- Coating & Electronics: As cross-linking agent, improves moisture resistance, dielectric stability for printed circuit boards
- Agrochemicals: Precursor for selective herbicides & plant growth regulators
- Metallurgy & Fluids: Temporary binders and modifiers for advanced water treatment chemicals
Advantages Over Conventional Intermediates:
- Ultra-low impurity profile (heavy metals ≤10 ppm)
- Product traceability (batch-specific QR)
- Extended shelf life (36+ months)
- Full compliance: ISO, ANSI, GOST
7. Manufacturer Comparison: Refine vs. Major Global Suppliers
| Criteria |
Refine N,N'-Dimethyl Urea |
Global Peer A (India) |
Global Peer B (Europe) |
| Purity (%) |
≥99.5 |
98.0-99.0 |
98.8 |
| Compliance |
ISO 9001:2015,
ANSI/ASTM,
FDA/EMA (optional) |
ISO 9001:2015 |
cGMP,
REACH |
| Stability (Months) |
36 |
18-24 |
24 |
| Supply Capability(t/y) |
900+ |
400 |
300 |
| Application Support |
API, Polymeric, Electronics,
Agrochemicals |
API only |
Polymer, API |
| Certification |
Full Traceable CoA, EN10204 3.1 |
Partial |
Full |
Certified to leading global pharma intermediates standards—Refine delivers a clear advantage in purity, shelf life, multi-industry support, and traceability.
8. Customization & Value-Added Solutions
- Tailored Grade & Particle Size: Custom micronization for polymer composites or API process optimization (D50: 100 to 400 µm)
- Packaging Flexibility: Bulk drums, flexible bags, nitrogen-flushed bags for O2/Moisture protection in special handling environments
- Regulatory Dossier Support: Includes full QC records, CoA, and third-party inspection on demand
- Supply Chain Integration: Just-in-time inventory, vendor managed inventory (VMI)
- Process Engineering Collaboration: Joint development of process parameters, on-site technical support for scale-up and qualification runs
9. Real-World Case Studies & User Feedback
Case 1: Large-Scale Japanese Pharmaceutical Manufacturer
- Problem: Existing dimethylurea supplier unable to achieve <0.2% moisture, risking hydrolytic degradation
- Solution: Refine supplied high-purity, vacuum-dried batch w/ full documentation (ISO, CoA, batch trace)
- Outcome: Elevated API yield by 7.8%, annualized batch rejection from 4.1% to 0.3%
Case 2: European Polymer Masterbatch Company
- Problem: Additive stability in UV aging tests was below specification at competitive supplier
- Solution: Custom Refine dimethylurea grade with enhanced antioxidant compatibility delivered
- Outcome: Long-term weatherability, compliance with ASTM/DIN polymer standards
User Feedback (2023-2024):
- “Batch-to-batch consistency is excellent, meets every cGMP and ISO spec for our pharma lines.” — Lead Chemist, Asia
- “Technical documentation and CoAs are supplied with complete traceability and transparency.” — Global Sourcing Lead, EU
10. FAQ — Professional Terms & Application Insights
Q1: What is the chemical structure of N,N'-Dimethyl Urea?
Answer N,N'-Dimethyl Urea is an amide derivative formed by replacing the hydrogen atoms on the urea molecule with methyl groups (structure: (CH3)2N-CO-NH2). This modification strengthens its solubility and reactivity in condensation reactions.
Q2: What are the typical specifications and tolerance levels for impurities in pharmaceutical-grade dimethylurea?
Answer Pharmaceutical grade: Purity ≥99.5%, Moisture ≤0.2%, Chloride/Sulfate ≤0.005%, Heavy metals ≤10 ppm. Strict control ensures no risk of undesired byproducts or toxicity in APIs.
Q3: Which standards govern the quality and handling of pharma intermediates?
Answer ISO 9001:2015 for quality management, ANSI for specific analytical tests, FDA/EMA cGMP for pharma use, and GOST R for Russia/CIS.
Q4: How are additives for polymers like dimethylurea incorporated into manufacturing?
Answer These additives are custom-formulated during melt or solution blending stages. Controlled particle size enables uniform dispersion increasing extension of polymer lifespan, UV stability, and colorfastness.
Q5: What testing methods are applied for impurity and stability determination?
Answer ISO/ASTM-compliant HPLC for organic impurities, GC-MS for volatile organics, XRF for heavy metals, and thermal cycling (EN60068) for shelf-life.
Q6: Is there a material difference for dimethylurea when applied to APIs versus non-pharma?
Answer Yes — pharma-intermediates use demands higher grades, controlled trace elements, and GMP compliance. Industrial additive grades can have broader impurity spectrum and different finish specifications.
Q7: What installation standards or handling guidelines are recommended?
Answer For pharmaceutical environments: cleanroom-level handling, antistatic storage, temperature- and humidity-controlled transport, and double-sealed packaging per ANSI/ASTM E29.
11. Delivery, Warranty, and Customer Support
- Supply Lead Time: Standard batch 7-14 days; custom grades 18–28 days ex-works. Global bulk sea/air shipping available.
- Warranty: 24 months product quality guarantee under standard conditions; batch certificate (CoA) provided with each shipment.
- Technical Support: Dedicated PhD chemists, 24h/5d technical hotline, and process troubleshooting included with B2B supply.
- Documentation: CoA, batch trace/QR, MSDS, and full ISO certification on delivery.
- After-Sales: Root-cause quality investigation, rapid claims handling, and annual customer audit support.
Ask for free samples with technical dossier for qualified B2B users.
12. References & Further Reading
-
Grand View Research, “Pharmaceutical Intermediates Market Size, Share & Trends Analysis Report”, https://www.grandviewresearch.com/industry-analysis/pharmaceutical-intermediates-market
-
Chemical Engineering News, "Innovations in API Intermediate Manufacturing," cen.acs.org/Pharma-intermediates-pick-steam
-
Pharmaceutical Online Forum, “Challenges in Sourcing GMP Pharma Intermediates”, pharmaceuticalonline.com/Challenges-in-sourcing-GMP-intermediates
-
"Role and Importance of Intermediates in Pharmaceuticals", Indian Pharmaceutical Association Journal, ipa-india.org/publications
-
“Polymer Additives: Market Dynamics & Trends”, Plastics Today Journal, plasticstoday.com/materials/polymer-additives-market-outlook
-
"ISO Certification for API and Intermediates Manufacturing", Quality Progress, ASQ, asq.org/quality-resources/iso-9001