Chlorhexidine Acetate is an extensively utilized, broad-spectrum antiseptic and disinfectant, serving pivotal roles in pharmaceutical, healthcare, and industry domains. With its superior biocidal properties and scalable production processes, Chlorhexidine Acetate is setting benchmarks for safety, efficacy, and reliability across medical, veterinary, and industrial hygiene applications.
1. Industry Overview & Recent Trends
The demand for Chlorhexidine Acetate is projected to grow at a CAGR of 6.2% (2022–2027), driven by surging needs for advanced antibacterial solutions within healthcare, food-processing, and water treatment sectors
(MarketsandMarkets, 2024).
Rapid regulatory compliance, increased awareness of hospital-acquired infection (HAI) prevention, and updates to global standards—such as
ISO 9001:2015 on quality management—further accelerate adoption.
Key Data: The global Chlorhexidine Acetate market size surpassed USD 120 million in 2023, with healthcare (61%), veterinary (19%), and industrial (20%) as core end-use sectors.
2. Product Profile & Technical Specifications
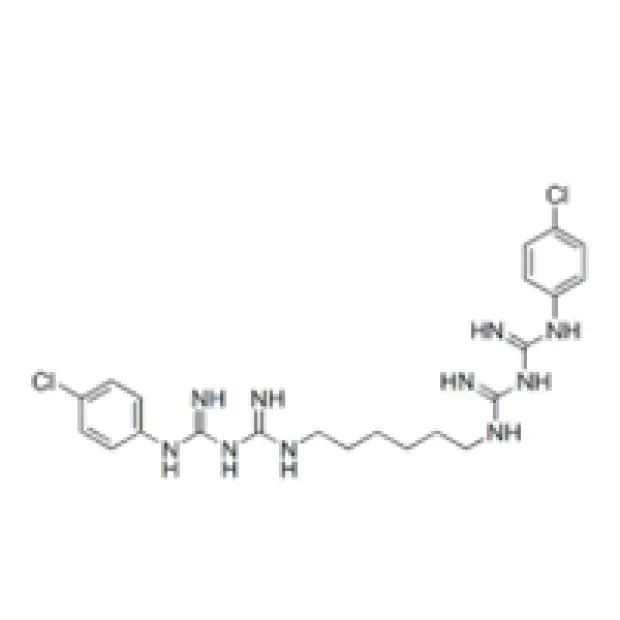
Chlorhexidine Acetate is a white to off-white crystalline powder, offering exceptional antimicrobial efficacy due to its cationic biguanide structure. Its unique physicochemical attributes facilitate versatile solutions for antiseptic, preservative, and industrial hygiene applications.
2.1 Chemical Identity & Key Properties
| Parameter |
Specification |
Standard/Reference |
| Chemical Name |
1,1'-Hexamethylenebis[5-(p-chlorophenyl)biguanide] diacetate |
IUPAC, USP |
| CAS Number |
56-95-1 |
NIH PubChem |
| Molecular Formula |
C26H38Cl2N10·2C2H4O2 |
USP |
| Molecular Weight |
625.58 g/mol |
USP |
| Appearance |
White or pale yellow crystalline powder |
Pharmacopeia |
| Purity |
≥ 98.0% |
USP, EP |
| pH (1% solution) |
5.5–7.0 |
Ph. Eur 10.0 |
| Solubility |
Freely soluble in water, alcohol; insoluble in ether |
USP 42 |
2.2 Technical Parameters At-A-Glance
2.3 Certifications & Quality Standards
- ISO 13485:2016—Quality management for medical device manufacturing
- USP/EP/BP—Compliant with global pharmacopeia
- FDA 510(k)—Medical use clearance (where applicable)
3. Manufacturing Process of Chlorhexidine Acetate
Flow Diagram – From Raw Material to High-Purity End Product:
1. Raw Materials
(p-Chloroaniline, Cyanoguanidine, Hexamethylene-bis-diamine)
→
2. Synthesis Reaction
(Biguanide condensation; 85–98℃, controlled pH)
→
3. Acetylation
(Acetate salt formation)
→
4. Crystallization & Purification
(Filtering, washing, drying)
→
5. Quality Control
(ISO, USP testing)
→
6. Packaging & Distribution
Key Technologies: Precision pH management, temperature control (CNC-process protocols), multi-stage crystallization, and in-situ QC inspection (in line with ISO 9001).
3.1 Material Selection & Processing Details
- Purity Assurance: All precursors ≥99.0% purity (GMP-certified sources)
- Crystallization: Multi-step filtration/washing removes residual impurities
- Final QC: HPLC, FTIR, and endotoxin tests; each batch tied to full CoA/documentation
- Compliance: Traceability matrix; meets ISO/ANSI process safety
4. Technical Advantages & Industry Positioning
4.1 Advantages over Competing Antiseptics
| Property |
Chlorhexidine Acetate |
Povidone-Iodine |
Ethanol |
Hydrogen Peroxide |
| Antimicrobial Spectrum |
Gram+/Gram-, fungi, enveloped viruses |
Broad, but tissue-irritant |
Mostly bacteria, no spores |
Bacteria, spores (low conc.), less persistent |
| Skin Irritation |
Very Low |
Moderate |
Moderate–High (drying) |
Low–Moderate |
| Residual Activity |
Up to 6 hours |
< 60 mins |
< 15 mins |
< 30 mins |
| Material Compatibility |
Excellent |
Discolors plastics/metals |
Attack plastics over time |
Oxidizes metals |
| Corrosion Resistance |
Very High |
Low |
Low |
Very Low |
| Cost Efficiency |
High (concentrated use) |
Moderate |
Moderate |
Low–Moderate |
4.2 Data Visualization: Usage Trends by Industry 2023
5. Leading Manufacturers: Benchmarking & Custom Solutions
Mainstream suppliers of Chlorhexidine Acetate vigorously compete in process control, purity, scaling, and regulatory response. The following table offers a concise comparison of select global providers:
| Manufacturer |
Certifications |
Purity (%) |
Batch Size (kg) |
Custom Solutions |
Key Industries Served |
| Hebei Guanxing Chemical |
ISO 9001, ISO 13485, FDA |
98.5–99.2 |
250–3000 |
Yes (particle size, packaging) |
Hospitals, Pharma, Water, Veterinary |
| BASF (Germany) |
ISO 9001, GMP |
98.0–99.0 |
200–2000 |
Yes (custom formulations) |
Medical, Lab, Industrial |
| Procos S.p.A. |
ISO 9001, DMF, CEP |
98.0–98.9 |
100–1200 |
Partial (packaging) |
API, Pharma |
Chlorhexidine Acetate from Hebei Guanxing Chemical stands out for traceable quality, scale-up flexibility, and robust compliance, offering custom solutions for both large bulk (tonnage) and low-impurity laboratory applications.
5.1 Customization Capabilities
- Particle Size Distribution: 90% < 63–125 μm (ultrafine available)
- Packaging: Wide range from 10kg barrier-drum to 800kg tote
- Formulation: Customized blends (e.g. synergic with surfactants or biocides)
- Labeling & Regulatory: GHS, CLP, MDR, REACH compliance
6. Application Scenarios & Case Studies
6.1 Typical Industrial & Medical Use Cases
- Hospital Disinfection: Preoperative skin prepping, instrument sterilization (CDC, 2022)
- Veterinary: Surgical wound cleaning for large and small animals
- Hand Sanitizers: Alcohol-free formula for frequent use (lower skin-impact)
- Industrial Water Treatment: Controls microbial biofilms in cooling towers, food processing pipelines
- Pharmaceutical Formulation: Dental gels, catheter flushes, topical creams
- Public Hygiene: Hand-wash stations, ophthalmic solutions
6.2 Experience Feedback & Success Cases
Case: Tertiary Care Hospital, Germany
"After switching to Chlorhexidine Acetate for surgical site prep, post-op infection rates fell by 28% within 12 months. Crucially, no adverse skin events were recorded, improving both patient safety and operational costs."
- Dr. Karl Schröder, Chief Infection Control
Case: Food Processing Plant, SE Asia
"With Chlorhexidine Acetate dosing, biofilm formation in pipes was reduced by over 90%, verified by total viable count (TVC) tests. The plant's overall maintenance downtime dropped by 15%."
- QA Manager Report 2023

7. Delivery, Warranty, and Support
- Lead Time: Standard 7–15 working days for stock; 3–5 weeks for custom batches
- Warranty: 24-month shelf life under recommended storage (15–25°C, dry, sealed)
- Support: 24/7 online technical & regulatory support; compliance documentation upon request
- Safety: Full GHS-compliant MSDS, safe transport per UN 3077
- Sampling: Pre-shipment and third-party testing support
8. Frequently Asked Technical Questions (FAQ)
Technical FAQ on Chlorhexidine Acetate
Q1. What is the recommended particle size for medical formulations?
A1. USP/EP-compliant medical applications specify D90 ≤ 110 μm to ensure rapid solubilization and optimal dispersion.
Q2. Which standards must the manufacturing process comply with?
A2. The process must adhere to ISO 9001/13485, Good Manufacturing Practice (GMP), and in some applications, the U.S. FDA 21 CFR 210/211 for pharmaceuticals.
Q3. How is product purity validated?
A3. Each batch is assessed via HPLC, IR spectroscopy, and residual solvents tested to USP 467 and ICH Q3C standards.
Q4. What is the shelf-life and optimal storage condition?
A4. The product retains >98% activity for up to 2 years when kept sealed, light-protected, and dry at 15–25°C, as per accelerated stability studies (ICH Q1A-R2).
Q5. Are there material compatibility concerns for industrial equipment?
A5. Chlorhexidine Acetate is compatible with stainless steel (304/316), HDPE, PVC, but not advisable for unprotected aluminum or non-polar elastomers.
Q6. What testing is performed for microbiological efficacy?
A6. Microbial kill rate validated by EN 1276 (bacteria), EN 13697 (fungi), and ASTM E2315, with data indicating ≥99.99% reduction of S. aureus in 30 seconds.
Q7. Can product packaging be customized for export markets?
A7. Yes, available packaging meets international requirements (UN, CLP, OSHA GHS labeling), with tamper-evident seals and full lot traceability.
9. References & Industry Resources
- MarketsandMarkets, "Chlorhexidine Acetate Market by Type & Application–Global Forecast 2027", Link
- CDC Guideline for Disinfection and Sterilization in Healthcare Facilities, Link
- ISO 13485:2016 Medical Devices—Quality Management Systems, Link
- PubChem Database: Chlorhexidine Acetate, Link
- Icar Journal, "Advances in Biguanide Antiseptics: A Review", Link
- Chemical Forums: Large-Scale Synthesis and QC of Chlorhexidine Salts, Link