
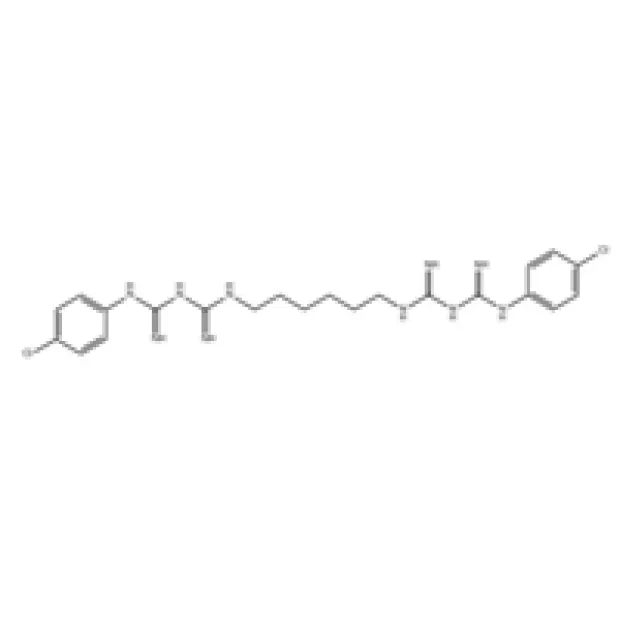
In the realm of advanced chemical solutions, Chlorhexidine stands out as a highly effective and widely utilized broad-spectrum antimicrobial agent. Its unique molecular structure, a cationic biguanide, enables it to exert potent antiseptic and disinfectant properties against a wide range of gram-positive and gram-negative bacteria, fungi, and some viruses. This efficacy, combined with its sustained residual activity, makes it an indispensable compound across numerous critical applications, ranging from sophisticated medical procedures to daily hygiene products. Our deep understanding of its chemical synthesis and application nuances positions us as a premier supplier, ensuring consistent quality and performance for discerning B2B clients globally. We consistently focus on delivering a product that meets rigorous international standards, thereby supporting the integrity and efficacy of our clients' end-use formulations.
The global market for antimicrobial agents, and particularly Chlorhexidine, is experiencing robust growth driven by escalating healthcare-associated infections (HAIs), increasing awareness regarding personal hygiene, and expanding applications in veterinary and cosmetic sectors. Projections indicate a significant compound annual growth rate (CAGR) for the coming years, fueled by stricter regulatory standards for infection control and a surge in demand for antiseptic products post-global health crises. This upward trend necessitates a reliable supply chain capable of delivering high-purity Chlorhexidine consistently. Furthermore, the push towards more sustainable and safer chemical manufacturing processes is shaping procurement decisions, with a preference for suppliers demonstrating strong environmental, social, and governance (ESG) commitments. Our strategic positioning allows us to cater to these evolving market demands, offering a product that not only performs optimally but is also produced responsibly.
The efficacy and safety of Chlorhexidine are directly correlated with its adherence to stringent technical specifications. As a key ingredient in pharmaceutical and medical formulations, parameters such as purity, solubility, and pH are critical for achieving desired antimicrobial action and ensuring product stability. Our Chlorhexidine undergoes rigorous quality control checks at every stage of its production to meet or exceed international pharmacopoeial standards, including USP (United States Pharmacopeia), BP (British Pharmacopoeia), and EP (European Pharmacopoeia). Below is a table outlining typical specifications for high-grade Chlorhexidine Digluconate solution, a commonly utilized form:
| Parameter | Specification | Test Method |
|---|---|---|
| Assay (as Chlorhexidine Digluconate) | 19.0% - 21.0% (w/v) | HPLC / Titration |
| Appearance | Clear, colorless or pale-yellow liquid | Visual Inspection |
| pH | 5.5 - 7.0 (1% w/v solution) | pH Meter |
| Related Substances | ≤ 0.5% (Total Impurities) | HPLC |
| Heavy Metals | ≤ 10 ppm | ICP-MS |
| Microbial Limits | Total Aerobic Microbial Count ≤ 100 CFU/ml; Absence of Specific Pathogens | USP <61> & <62> |
These detailed specifications ensure that our Chlorhexidine is not only effective but also safe for its intended high-stakes applications.
The production of high-quality Chlorhexidine is a multi-stage chemical synthesis process that demands precision, stringent quality control, and adherence to Good Manufacturing Practices (GMP). Our manufacturing facility utilizes state-of-the-art reactors and purification systems to ensure optimal yield and unparalleled purity. The typical process begins with carefully selected raw materials, which undergo initial quality verification. These raw materials are then reacted under controlled conditions, often involving specific temperatures, pressures, and catalysts to form the desired Chlorhexidine base.
Following the initial synthesis, the crude product undergoes a series of purification steps, including crystallization, filtration, and drying. These stages are critical for removing impurities and achieving the high assay levels required for pharmaceutical and medical applications. The final product is then converted into its salt forms, such as Chlorhexidine Digluconate or Chlorhexidine Acetate, depending on specific customer requirements, by reaction with the respective acids. Each batch is subjected to comprehensive analytical testing, including chromatography (HPLC), spectroscopy (UV-Vis), and microbiological assays, to confirm purity, concentration, and the absence of contaminants. Our processes are ISO 9001 certified, reflecting our commitment to global quality management standards, and are designed to extend the product's shelf life and stability, typically ensuring a minimum of 24-36 months of validated efficacy when stored under recommended conditions. This meticulous approach ensures that our Chlorhexidine maintains its powerful antimicrobial properties throughout its lifecycle, delivering superior performance in critical environments such as hospitals, pharmaceutical production facilities, and veterinary clinics.
The broad-spectrum antimicrobial activity and excellent safety profile of Chlorhexidine make it a versatile agent across a multitude of industries. In the healthcare sector, it is foundational for surgical scrubs, skin preparation before invasive procedures, and as an active ingredient in antiseptic wound cleansers, significantly reducing the risk of surgical site infections (SSIs) and hospital-acquired infections (HAIs). Its efficacy in disrupting microbial cell membranes provides a lasting protective barrier on the skin, a key advantage in clinical settings.
Beyond direct medical applications, Chlorhexidine is a staple in oral care products, including mouthwashes and toothpastes, effectively combatting gingivitis, plaque formation, and maintaining overall oral hygiene. The veterinary industry utilizes it extensively for pet skin infections, surgical site disinfection, and ear cleansers. Furthermore, its inclusion in cosmetic formulations, such as hand sanitizers and specialized skin care products, is growing due to increasing consumer demand for effective microbial control. Its consistent performance across these varied and demanding environments underscores its reliability and critical importance.
Our commitment to delivering superior Chlorhexidine is rooted in several technical advantages that set our product apart. Firstly, its broad-spectrum efficacy ensures comprehensive protection against a wide array of pathogens, simplifying formulation development for our clients. Secondly, Chlorhexidine exhibits remarkable persistence; it binds to the skin and mucous membranes, providing prolonged antimicrobial activity even after application, which is crucial for sustained infection control. This substantive effect is a distinct advantage over many alcohol-based disinfectants that evaporate quickly.
Thirdly, its low systemic absorption and favorable safety profile, when used as directed, make it suitable for frequent and long-term use in various sensitive applications. Our product’s consistent purity, verified through rigorous analytical methods, translates into enhanced formulation stability and predictable performance in end-products. Clients benefit from a reduced risk of adverse reactions and improved overall product effectiveness. This combination of efficacy, persistence, and safety positions our Chlorhexidine as the preferred choice for demanding B2B applications where performance cannot be compromised.
Selecting the right Chlorhexidine manufacturer is paramount for ensuring the quality and regulatory compliance of your end-products. Key criteria for evaluation include the manufacturer’s adherence to Good Manufacturing Practices (GMP) and ISO certifications, which are foundational indicators of a robust quality management system. A reputable supplier will provide comprehensive documentation, including Certificates of Analysis (CoA), Material Safety Data Sheets (MSDS), and regulatory support dossiers.
Furthermore, assess the manufacturer’s experience in the chemical industry, their capacity for consistent supply, and their commitment to sustainable production practices. We distinguish ourselves through our unwavering dedication to these principles, backed by years of experience serving the pharmaceutical, medical device, and personal care industries. Our manufacturing processes are routinely audited by third-party bodies and our own internal quality assurance teams to maintain the highest standards. We invite potential partners to review our certifications and engage in technical discussions to understand our capabilities fully, ensuring a transparent and trustworthy supplier relationship.
Recognizing that each client's formulation and application may have unique requirements, we offer bespoke solutions for Chlorhexidine supply. This includes varying concentrations of Chlorhexidine Digluconate solution, different salt forms (e.g., acetate or hydrochloride), and specific packaging options to integrate seamlessly into your production lines. Our technical team is equipped to collaborate closely with your R&D departments, providing expert guidance on formulation compatibility, stability testing, and regulatory considerations.
Whether you require a specific purity threshold for a novel pharmaceutical or bulk quantities for high-volume consumer products, our adaptable manufacturing capabilities ensure that your precise needs are met without compromising on quality or consistency. This client-centric approach has fostered long-term partnerships, enabling our customers to innovate with confidence, knowing they have a reliable and flexible supplier for their Chlorhexidine requirements.
Our high-quality Chlorhexidine has been instrumental in numerous success stories across diverse industries. For instance, a leading pharmaceutical company successfully launched a new line of surgical skin preparation solutions, achieving superior antimicrobial efficacy and patient safety, directly attributed to the consistent purity and reliable supply of our Chlorhexidine Digluconate. Their clinical trials demonstrated a significant reduction in post-operative infection rates compared to previous formulations.
In the oral care segment, our Chlorhexidine was integrated into a popular mouthwash brand, which subsequently reported enhanced plaque reduction and gingivitis control among users, leading to increased market share. A veterinary clinic chain standardized their disinfectant protocols using formulations containing our Chlorhexidine, resulting in a demonstrable decrease in cross-contamination and an improvement in overall animal health outcomes. These real-world applications underscore the tangible benefits and trusted performance of our product, solidifying our reputation as a preferred supplier for critical antimicrobial solutions.
A1: We primarily supply Chlorhexidine Digluconate solution (typically 20% w/v), which is the most common and versatile form. We can also provide Chlorhexidine Acetate and Chlorhexidine Hydrochloride in powder forms upon specific request, subject to availability and minimum order quantities.
A2: Our standard delivery period for Chlorhexidine varies depending on order volume and destination. Generally, lead times range from 2 to 4 weeks after order confirmation. We maintain robust inventory levels and efficient logistics to minimize delays and accommodate urgent requirements whenever possible. Specific lead times will be confirmed upon quotation.
A3: Yes, our Chlorhexidine products are manufactured under strict GMP guidelines and comply with key international pharmacopoeial standards, including USP, BP, and EP. We provide comprehensive regulatory documentation to support your product registrations and compliance needs.
A4: We offer extensive technical support, including assistance with formulation challenges, guidance on product stability, compatibility advice, and detailed data sheets. Our team of chemical experts is available for consultations to ensure optimal integration of our Chlorhexidine into your specific applications.
Our commitment extends beyond product quality to encompass a seamless customer experience, from order placement to delivery and beyond. We operate a highly efficient global logistics network to ensure timely and secure delivery of Chlorhexidine to your facilities worldwide. Each shipment is meticulously packed and handled to preserve product integrity throughout transit. Our average delivery cycle is optimized to meet the dynamic demands of the B2B market, providing clear communication on transit times and tracking information.
We offer a comprehensive quality assurance pledge, guaranteeing that every batch of Chlorhexidine shipped adheres to the agreed-upon specifications and international standards. Our robust quality management system, backed by ISO 9001 certification and adherence to GMP, underpins this commitment. In the unlikely event of any concerns, our dedicated customer support team is readily available to provide prompt assistance, resolve issues efficiently, and ensure complete client satisfaction. We stand by our products with a transparent warranty policy that reflects our confidence in their superior quality and performance, fostering long-term, trust-based partnerships.
As a cornerstone antimicrobial agent, Chlorhexidine continues to play a vital role in global health and hygiene. Our expertise in its manufacturing, coupled with an unwavering commitment to quality, technical excellence, and client-focused service, positions us as the ideal partner for your Chlorhexidine supply needs. We are dedicated to providing a product that not only meets but exceeds industry benchmarks, contributing directly to the efficacy and success of your formulations. Partner with us to leverage high-purity Chlorhexidine and benefit from a reliable, expert-driven supply chain.
This is the first article
Hebei Guangxing Chemical: Integrating into the Digital Economy Ecosystem with innovative Power
If you are interested in our products, you can choose to leave your information here, and we will be in touch with you shortly.