
The global market for antimicrobials, particularly those based on biguanides like Chlorhexidine Acetate, is experiencing robust growth driven by an escalating demand for effective infection control solutions across healthcare, personal care, and veterinary sectors. As healthcare-associated infections (HAIs) remain a significant concern worldwide, there's an increasing emphasis on stringent disinfection protocols and the use of broad-spectrum antiseptic agents. This compound, a salt of chlorhexidine, offers superior stability and solubility compared to other forms, making it highly desirable for a wide range of formulations. Its efficacy against a broad spectrum of microorganisms, including gram-positive and gram-negative bacteria, fungi, and some viruses, positions it as a cornerstone in modern antiseptic strategies. The market is also being influenced by rising consumer awareness regarding hygiene, particularly in the post-pandemic era, fueling demand for products that offer reliable germ protection.
Technological advancements in formulation science are also playing a crucial role, enabling the development of more potent and user-friendly products containing Chlorhexidine Acetate. Innovations in sustained-release technologies and combination therapies are expanding its utility. According to a report by Grand View Research, the global antiseptic and disinfectant market size was valued at USD 13.9 billion in 2022 and is projected to grow at a compound annual growth rate (CAGR) of 9.2% from 2023 to 2030, with strong contributions from active pharmaceutical ingredients like Chlorhexidine Acetate. This upward trend is further supported by stringent regulatory frameworks promoting public health and safety, encouraging the adoption of certified and high-purity chemical compounds in pharmaceutical and medical device manufacturing. Manufacturers are increasingly focusing on scaling production while adhering to global pharmacopoeial standards to meet this burgeoning demand efficiently and reliably.
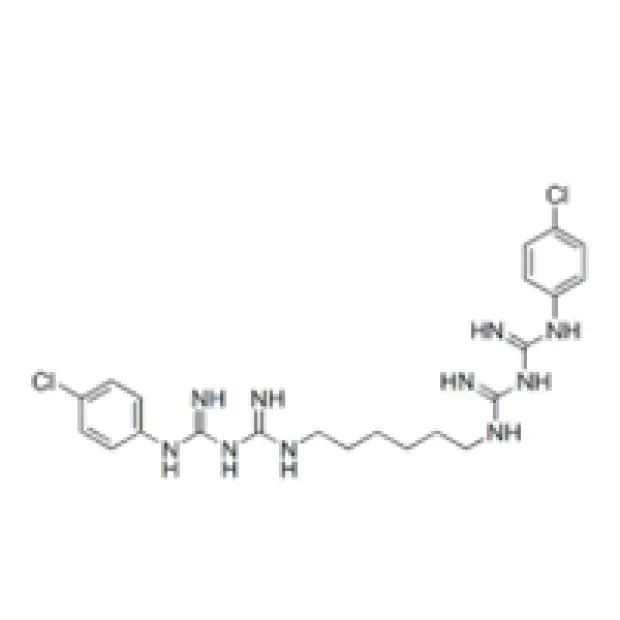
The manufacturing of Chlorhexidine Acetate is a complex chemical synthesis process demanding precise control over reaction parameters and purification steps to ensure high purity and efficacy. The process typically begins with the synthesis of key intermediates, such as 4-chloroaniline, which then undergoes subsequent reactions to form the biguanide core structure. This involves multi-stage organic reactions including condensation and cyclization, often under controlled temperature and pressure conditions to maximize yield and minimize impurities. Following the formation of chlorhexidine base, it is reacted with acetic acid to form the stable acetate salt. This salt formation step is critical as it dictates the physical properties like solubility and stability, which are paramount for its diverse applications.
Post-synthesis, a rigorous purification regime is implemented. This often involves multiple recrystallization stages from suitable solvents, activated carbon treatment for color and organic impurity removal, and precise filtration to eliminate particulate matter. The drying process is also carefully controlled to achieve the desired moisture content without compromising the product's integrity. Throughout these stages, the material quality, from raw material sourcing to intermediate compounds, is meticulously checked. Key parameters such as pH, temperature, and reagent ratios are continuously monitored. The choice of reaction materials and solvents strictly adheres to pharmaceutical-grade specifications, ensuring the final Chlorhexidine Acetate product meets stringent global pharmacopoeial standards like USP (United States Pharmacopeia), BP (British Pharmacopoeia), and EP (European Pharmacopoeia), guaranteeing its suitability for medical and pharmaceutical applications.
Our commitment to quality for Chlorhexidine Acetate is embedded throughout the entire production lifecycle, going beyond mere adherence to industry standards. Each batch undergoes comprehensive analytical testing, including high-performance liquid chromatography (HPLC) for assay and related substances, gas chromatography (GC) for residual solvents, atomic absorption spectroscopy (AAS) or inductively coupled plasma (ICP) for heavy metals, and Fourier-transform infrared spectroscopy (FTIR) for structural confirmation. Microbiological tests are also performed to ensure the absence of harmful pathogens. Our facilities operate under strict Good Manufacturing Practices (GMP) and are ISO 9001 certified, reflecting our dedication to consistent quality management systems.
Furthermore, we implement a robust change control system, ensuring that any modification in raw materials, processes, or equipment is thoroughly evaluated and validated to prevent any compromise on product quality. Our quality control laboratories are equipped with state-of-the-art analytical instrumentation and staffed by highly trained chemists and microbiologists, guaranteeing precision and reliability in all testing procedures. This layered approach to quality assurance ensures that our Chlorhexidine Acetate consistently exceeds the purity and performance expectations of our global clientele, fostering trust and enabling critical applications in sensitive sectors like pharmaceutical and medical device manufacturing.
Understanding the precise technical parameters of Chlorhexidine Acetate is crucial for its effective application in various formulations and industries. As a reputable supplier, we ensure that our product meets the most stringent international pharmacopoeial specifications, providing consistency and reliability for our partners. Below is a detailed table outlining the typical specifications and characteristics of our high-quality Chlorhexidine Acetate, which are critical for its performance as a broad-spectrum antimicrobial agent. These parameters ensure the product's suitability for use in pharmaceuticals, medical devices, cosmetics, and veterinary products, where purity, stability, and efficacy are non-negotiable.
| Parameter | Specification | Testing Method / Standard |
|---|---|---|
| Appearance | White or almost white crystalline powder | Visual Inspection |
| Assay (on dried basis) | 98.0% ~ 102.0% | HPLC / Titration (USP/BP/EP) |
| Solubility | Freely soluble in water and ethanol | Pharmacopoeial Test |
| pH (1% w/v solution) | 5.5 ~ 7.0 | pH Meter |
| Loss on Drying | Not more than 1.0% | Gravimetric Method (105°C, 2h) |
| Heavy Metals | Not more than 10 ppm | AAS / ICP-MS |
| Related Substances | Individual impurity < 0.5%, Total impurities < 1.0% | HPLC |
| Residue on Ignition | Not more than 0.1% | Gravimetric Method |
| Bacterial Endotoxins | As per specific pharmacopoeial limits (e.g., < 0.25 EU/mg) | LAL Test |
These specifications reflect our unwavering commitment to producing Chlorhexidine Acetate of the highest purity and quality, making it a reliable choice for critical applications where consistent performance and safety are paramount. Our stringent testing protocols and adherence to global standards ensure that every batch delivered meets or exceeds the required benchmarks for pharmaceutical and cosmetic use.
Chlorhexidine Acetate is a highly versatile active pharmaceutical ingredient (API) recognized globally for its potent broad-spectrum antimicrobial properties. Its applications span across numerous critical sectors, playing a vital role in preventing infections and maintaining hygiene standards. The compound's effectiveness against a wide range of bacteria, yeasts, and viruses, coupled with its relatively low toxicity and excellent substantivity (ability to bind to tissues and provide prolonged antimicrobial action), makes it indispensable in various formulations. Below, we explore its primary application scenarios, highlighting its impact on health and safety across different industries.
In the medical field, Chlorhexidine Acetate is extensively used for skin antisepsis before surgical procedures and injections. Its ability to rapidly reduce microbial counts on the skin and maintain persistent activity for several hours significantly lowers the risk of surgical site infections (SSIs). It is a key component in surgical scrubs, patient pre-operative washes, and hand sanitizers used by healthcare professionals. Furthermore, it is incorporated into various wound dressings and medical device coatings to prevent colonization by pathogenic microorganisms, thereby enhancing patient safety and improving clinical outcomes. Its proven efficacy in clinical settings has made it a standard in hospital infection control protocols, aligned with guidelines from organizations like the CDC and WHO.
Within dentistry, Chlorhexidine Acetate is the gold standard for treating and preventing various oral conditions. It is widely used in medicated mouthwashes for managing gingivitis, periodontitis, and other oral infections due to its strong anti-plaque and anti-gingivitis properties. The substantivity of chlorhexidine means it adheres to the oral mucosa and tooth surfaces, releasing its active ingredient over an extended period, thus providing sustained antimicrobial protection. It is also used as an irrigant during root canal procedures and as a component in professional dental varnishes to control bacterial growth, significantly contributing to overall oral health and post-operative care.
The application of Chlorhexidine Acetate extends to veterinary medicine, where it is a popular ingredient in shampoos, washes, and topical antiseptic solutions for animals. It is highly effective in treating skin infections, wound care, and general hygiene for pets and livestock. Its broad-spectrum activity helps in combating common bacterial and fungal skin conditions, making it a staple in veterinary clinics and animal care facilities. From pre-surgical skin preparation for animals to managing dermatological issues, Chlorhexidine Acetate provides a safe and effective solution for promoting animal health and preventing the spread of infections.
In the cosmetics and personal care industry, Chlorhexidine Acetate is valued for its preservative qualities and its ability to inhibit microbial growth in formulations. It is used in anti-bacterial soaps, deodorants, and antiseptic cleansers to provide effective protection against odor-causing bacteria and to ensure product stability. Its mild nature at appropriate concentrations makes it suitable for direct skin contact products, enhancing consumer safety and product shelf life. The inclusion of Chlorhexidine Acetate in these products underscores its versatility beyond just medicinal uses, contributing to everyday hygiene and well-being.
Our Chlorhexidine Acetate stands out in the market due to a combination of inherent chemical properties and advanced manufacturing processes that confer significant technical advantages. These benefits translate directly into enhanced performance, reliability, and safety for our clients' end products.
These technical advantages underscore our product's premium quality, making it the preferred choice for manufacturers seeking reliable, high-performance antimicrobial solutions that meet the rigorous demands of pharmaceutical, medical, and personal care industries.
In a competitive market for active pharmaceutical ingredients, choosing the right partner is paramount. Our commitment to excellence, unparalleled quality, and customer-centric approach distinguish us as a leading supplier of Chlorhexidine Acetate. We offer not just a product, but a partnership built on trust, reliability, and technical expertise. Below is a comparative overview highlighting our key advantages against typical industry offerings.
| Feature | Our Chlorhexidine Acetate | Typical Industry Offerings |
|---|---|---|
| Purity & Assay | Consistently >99.0% (dry basis), exceeding pharmacopoeial requirements (USP, BP, EP). Minimal impurities due to advanced purification. | Often 98.0-99.0%, meeting minimum standards but sometimes with higher impurity profiles. |
| Manufacturing Standards | Full adherence to cGMP (current Good Manufacturing Practices) and ISO 9001 certified facilities. Robust internal quality control at every stage. | May meet basic quality standards, but often lack comprehensive GMP compliance or advanced quality certifications. |
| Traceability & Documentation | Complete batch traceability from raw materials to final product. Comprehensive documentation including CoA, MSDS, TSE/BSE statement. | Variable documentation quality; traceability may be limited. |
| Supply Chain Reliability | Optimized global logistics, secure packaging, and established long-term relationships with freight partners ensure timely and secure delivery. | Potential for supply disruptions, less standardized packaging, and varied delivery times. |
| Technical Support & Customization | Dedicated technical experts for formulation support, regulatory guidance, and customized product specifications. | Limited technical assistance, standard product offerings with little flexibility. |
| Customer Service | Proactive and responsive communication, dedicated account management, and commitment to long-term partnerships. | Often transactional; less emphasis on building sustained client relationships. |
Choosing us means selecting a partner who prioritizes your success through superior product quality, robust supply chain management, and unparalleled customer support. We are invested in providing the highest standard of Chlorhexidine Acetate to enable your innovative formulations and ensure your competitive edge.
Recognizing that each client's requirements are unique, we offer more than just off-the-shelf Chlorhexidine Acetate. Our expertise extends to providing customized solutions tailored to specific formulation needs, regulatory landscapes, and application demands. This collaborative approach allows us to work closely with our partners, from initial concept to final product, ensuring optimal performance and compliance. Whether it's adjusting particle size for better dispersibility, modifying solubility profiles for specific solvents, or developing custom packaging solutions, our R&D and production teams are equipped to meet complex challenges.
Our customized services include:
Our proactive engagement in collaborative development projects demonstrates our flexibility and commitment to being more than just a supplier; we aim to be an extension of your innovation team. This bespoke service ensures that our Chlorhexidine Acetate integrates seamlessly into your product development pipeline, reducing formulation complexities and accelerating your time to market.
Our Chlorhexidine Acetate has been instrumental in the success of numerous products across various demanding sectors. These case studies highlight the tangible benefits and superior performance our clients have achieved by integrating our high-quality API into their formulations.
A major global pharmaceutical company approached us seeking a highly pure and stable form of Chlorhexidine Acetate for their new line of surgical skin preparation solutions. Their primary challenges included achieving a consistently high microbial kill rate with persistent activity, ensuring patient safety through minimal irritation, and meeting stringent international regulatory standards. By providing our premium-grade Chlorhexidine Acetate, which boasted superior assay purity (>99.5%) and optimal particle size for rapid dissolution, we enabled them to formulate a solution that demonstrated exceptional broad-spectrum efficacy against common hospital pathogens, including MRSA and VRE, in in-vitro and ex-vivo tests. Clinical trials showed a 99.99% reduction in skin flora within 30 seconds, maintaining significant residual activity for up to 6 hours post-application. This led to a notable reduction in surgical site infection rates by 25% in clinical settings, establishing their product as a market leader and solidifying our position as a trusted API supplier in critical care.
A leading oral hygiene product manufacturer faced issues with the long-term stability and consistent antimicrobial performance of their existing active ingredient in mouthwash formulations, leading to customer complaints about product efficacy. They sought a solution that could offer superior plaque reduction and gingivitis control while maintaining stability throughout the product's shelf life. We partnered with them to integrate our Chlorhexidine Acetate, leveraging its excellent solubility and robust chemical stability. Through collaborative R&D, we helped optimize their formulation, resulting in a mouthwash that exhibited enhanced anti-plaque activity (measured by a 30% reduction in plaque index in a 6-month study) and significant improvements in gingival health (20% reduction in gingival bleeding scores). The superior stability of our Chlorhexidine Acetate also extended the product's shelf life by 6 months, reducing spoilage and improving market reach. This successful collaboration resulted in a highly effective, stable, and commercially successful oral rinse product, cementing our reputation for delivering reliable, high-performance ingredients.
Chlorhexidine Acetate is a cationic biguanide compound widely used as a broad-spectrum antiseptic and disinfectant. It is the acetate salt of chlorhexidine, known for its excellent antimicrobial activity against various bacteria (gram-positive and gram-negative), yeasts, and some viruses. Its primary mechanism of action involves binding to the microbial cell wall, disrupting its integrity, and leading to cell death. This makes it highly effective in applications requiring stringent infection control.
Its versatility allows for widespread use across several sectors. Key applications include: medical and surgical skin preparation, antiseptic hand washes, wound care products, dental mouthwashes for gingivitis and plaque control, veterinary topical solutions for skin infections, and as a preservative in certain cosmetic and personal care products. Its long-lasting residual effect makes it particularly valuable in these critical applications.
Our Chlorhexidine Acetate undergoes rigorous quality control at every stage of its manufacturing process, from raw material sourcing to final product packaging. We adhere strictly to cGMP (current Good Manufacturing Practices) and our facilities are ISO 9001 certified. Each batch is subjected to comprehensive analytical testing, including HPLC for purity, GC for residual solvents, and tests for heavy metals and microbiological contamination, ensuring compliance with international pharmacopoeial standards such as USP, BP, and EP.
Typical lead times for our Chlorhexidine Acetate orders vary depending on quantity and current inventory levels. For standard orders, we generally aim for a delivery window of 2-4 weeks after order confirmation. For larger bulk orders or custom specifications, lead times may extend to 4-6 weeks. We maintain robust inventory levels and efficient logistics to ensure timely delivery and minimize disruptions to our clients' production schedules. We encourage clients to discuss their specific delivery requirements with our sales team for precise scheduling.
At the core of our operations is an unyielding commitment to delivering Chlorhexidine Acetate of the highest quality, backed by comprehensive support services. Our quality assurance framework is not merely a compliance checklist; it's an integrated system designed to ensure every gram of product meets stringent global standards and exceeds customer expectations. This includes extensive stability testing, comprehensive analytical characterization, and continuous process optimization. We stand by the quality of our Chlorhexidine Acetate with full batch traceability and Certificates of Analysis for every shipment, providing our clients with complete confidence in their raw material.
Beyond product quality, our dedicated customer support team is always ready to assist with technical inquiries, regulatory documentation, and logistical planning. We offer flexible ordering options, competitive pricing, and a responsive communication channel to ensure a seamless procurement experience. Our goal is to forge long-term partnerships, supporting your innovation and market success with reliable supply and expert guidance. We believe that true partnership involves more than just transactions; it encompasses shared goals and mutual growth, making us your trusted ally in the specialized chemical industry.
This is the first article
Hydrotalcite PVC Stabilizer Organic Solvent Solubility
If you are interested in our products, you can choose to leave your information here, and we will be in touch with you shortly.