
The global animal health sector is experiencing unprecedented growth, driven by escalating demand for animal-derived protein, heightened awareness regarding zoonotic diseases, and significant advancements in veterinary medicine. Within this dynamic landscape, the persistent challenge of parasitic diseases, particularly tick-borne ailments like theileriosis, underscores the critical demand for highly effective antiprotozoal agents. Buparvaquone stands out as a cornerstone treatment in this specialized therapeutic area, celebrated for its potent efficacy against various Theileria species, which are responsible for immense economic losses in livestock industries across endemic regions in Africa, Asia, and the Middle East. The market trends indicate a consistent upward trajectory for veterinary pharmaceuticals, with a particular emphasis on sustainable, efficacious solutions that minimize disease burden, enhance animal welfare, and ultimately contribute to global food security. As livestock producers worldwide strive for optimal herd health and productivity in the face of climate change and emerging disease patterns, the adoption of advanced parasiticides like Buparvaquone becomes imperative. This necessitates navigating evolving regulatory frameworks, ensuring drug safety, and addressing the ongoing challenge of emerging drug resistance. Furthermore, the increasing integration of digital health solutions, genetic selection, and precision livestock farming practices is profoundly influencing the distribution and application strategies for such critical veterinary inputs, demanding robust and transparent supply chains alongside comprehensive technical support from manufacturers. The shift towards preventative health and integrated disease management programs further solidifies the long-term importance of reliable anti-theileriosis treatments, positioning Buparvaquone as a vital component in modern animal health strategies. Its pivotal role directly contributes to the global food security agenda by safeguarding livestock assets, which are foundational to many agricultural economies. The sustained need for high-purity, stable, and readily available active pharmaceutical ingredients (APIs) is paramount to meet this growing global demand, compelling manufacturers to continuously innovate in synthesis methodologies and quality assurance protocols to maintain consistency and reliability in every batch produced for the global market.
The manufacturing of Buparvaquone is a highly specialized chemical synthesis process, demanding unparalleled precision and stringent quality control at every stage to ensure the final product meets the highest pharmaceutical standards for veterinary use. Unlike the mechanical manufacturing processes (e.g., casting, forging, CNC machining) often associated with industrial components, the production of a complex chemical compound like Buparvaquone focuses intensely on molecular purity, structural integrity, and isomer control. The process typically initiates with the meticulous selection and procurement of high-purity precursor chemicals, such as 2-hydroxy-3-(3-methyl-2-butenyl)-1,4-naphthoquinone and tert-butyl bromide, which serve as foundational building blocks. The core synthesis involves a series of multi-step chemical reactions, often including specific alkylation and condensation reactions, where the tert-butyl group is strategically introduced onto the naphthoquinone core. These reactions are conducted under precisely controlled conditions of temperature, pressure, and pH, frequently requiring inert atmospheres, specific catalysts, and sophisticated reaction monitoring systems to optimize reaction yield, ensure selectivity, and minimize the formation of undesirable impurities or by-products. Following the primary synthesis, extensive purification steps are critically important to achieve the required pharmaceutical grade. This typically involves multiple rounds of recrystallization from optimized solvent systems, activated charcoal treatment for decolorization and impurity adsorption, and precise filtration techniques to remove any solid contaminants. The purity and identity of intermediate and final products are rigorously monitored using advanced analytical techniques, including High-Performance Liquid Chromatography (HPLC) for assay and related substances, Nuclear Magnetic Resonance (NMR) spectroscopy for structural elucidation, Infrared (IR) spectroscopy for functional group confirmation, and Mass Spectrometry (MS) for molecular weight verification. Compliance with international inspection standards such as Good Manufacturing Practices (GMP) and adherence to pharmacopeial monographs (e.g., British Pharmacopoeia, European Pharmacopoeia) are non-negotiable, underpinning every stage of production to guarantee the active pharmaceutical ingredient's safety, quality, and consistent efficacy for its intended veterinary application. This sophisticated and meticulous approach to synthesis ensures that each batch of Buparvaquone provides reliable, consistent therapeutic outcomes, safeguarding animal health globally and reinforcing trust in the product's performance and stability throughout its declared shelf life.
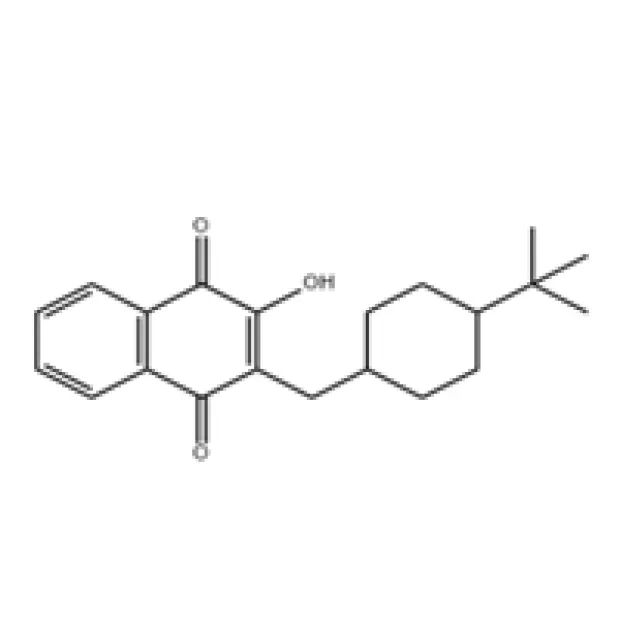
Figure 1: Conceptual representation of advanced chemical synthesis pathways for active pharmaceutical ingredients, emphasizing purity and control.
The superior performance of Buparvaquone in veterinary applications stems directly from its optimized technical parameters and inherent pharmacological advantages, making it an indispensable tool in the fight against theileriosis. As a 2-hydroxy-3-(substituted alkyl)-1,4-naphthoquinone derivative, its unique molecular structure is specifically designed for high efficacy against both the schizont and merozoite stages of Theileria species, which are critical for interrupting the parasite's life cycle within the host. Typical technical specifications for high-grade Buparvaquone API include a minimum purity level usually exceeding 98.5% as determined by High-Performance Liquid Chromatography (HPLC), a characteristic appearance ranging from white to yellowish crystalline powder, a precise melting point range typically between 105-109°C, and specific solubility characteristics (e.g., practically insoluble in water but freely soluble in common organic solvents like methanol or dichloromethane). These rigorous parameters are crucial for ensuring the drug's stability, facilitating accurate formulation into finished products, enabling precise dosing, and guaranteeing consistent therapeutic outcomes under diverse field conditions. The primary technical advantage of Buparvaquone lies in its potent antitheilerial activity and its remarkable ability to rapidly resolve clinical signs of the disease, with significant improvement often observed within 24-48 hours post-administration. Its favorable pharmacokinetic profile, including a relatively long half-life, contributes to sustained therapeutic concentrations in target tissues, thereby requiring fewer doses and simplifying treatment regimens for livestock farmers and veterinarians. Furthermore, it exhibits a broad spectrum of activity against various virulent Theileria species, including Theileria parva (the causative agent of East Coast fever), T. annulata (tropical theileriosis), and T. mutans. The safety profile of Buparvaquone, when administered correctly according to veterinary guidelines, is also a significant advantage, with minimal reported adverse effects in target species, ensuring animal welfare during treatment. This robust efficacy, combined with a favorable safety margin, makes it an indispensable tool in regions where theileriosis is endemic, safeguarding livestock populations and contributing significantly to the economic viability and sustainability of cattle farming globally. The product's inherent stability under various storage conditions further ensures a reliable supply chain, a crucial aspect for global distribution, reinforcing why Buparvaquone remains a preferred choice for combating one of the most economically devastating diseases in cattle.
| Parameter | Specification | Method/Remarks |
|---|---|---|
| Appearance | White to Yellowish Crystalline Powder | Visual Inspection |
| Assay (on dried basis) | ≥ 98.5% | HPLC |
| Related Substances (Total) | ≤ 1.0% | HPLC, per Pharmacopeia |
| Melting Point | 105-109°C | Capillary Method, USP/BP/EP |
| Loss on Drying | ≤ 0.5% | Gravimetry (105°C, 2h) |
| Residue on Ignition / Sulfated Ash | ≤ 0.1% | Pharmacopeial Method |
| Solubility | Practically insoluble in water; freely soluble in methanol or dichloromethane. | Visual Observation at 25°C |
| Heavy Metals | ≤ 10 ppm | ICP-MS/AAS |
The practical utility of Buparvaquone spans a diverse array of critical application scenarios within the animal health sector, directly impacting livestock productivity and farmer livelihoods. Primarily, it is an indispensable agent in regions heavily burdened by tick-borne parasitic diseases, especially tropical and East Coast fever in cattle, which devastate herds across sub-Saharan Africa, parts of Asia, and the Middle East. This includes its use in large-scale commercial dairy and beef farms, vital smallholder livestock operations in developing economies where cattle represent primary assets, and within governmental or non-governmental initiatives focused on controlling widespread animal diseases as part of national health programs. Veterinarians typically administer Buparvaquone for both the targeted treatment of clinical cases of theileriosis and as part of strategic control programs aimed at reducing disease incidence and transmission at a herd level. Its high efficacy translates into significant economic advantages for farmers by minimizing acute mortality rates, drastically reducing production losses (e.g., severe milk yield reduction, stunted growth in young stock, loss of draught power), and accelerating animal recovery to pre-disease productivity levels. Choosing the right manufacturer for Buparvaquone API is paramount, given the direct impact on animal health and producer profitability. Key differentiators among reputable manufacturers include unwavering adherence to stringent Good Manufacturing Practices (GMP) and relevant pharmacopeial standards (BP, EP, USP if applicable), robust demonstrated product stability and shelf-life data under various climatic zones, and comprehensive analytical Certificates of Analysis (CoA) provided for each batch, ensuring full traceability and compliance. Beyond core product quality, the manufacturer's service reliability, depth of technical support, and proven ability to offer customized solutions significantly influence purchasing decisions. For instance, leading suppliers can provide Buparvaquone in various packaging sizes and purities to suit different operational scales, from bulk quantities for large-scale pharmaceutical formulators producing injectable solutions to smaller, pre-weighed aliquots for specific research and development needs or small-batch formulation. Additionally, experienced manufacturers offer invaluable technical assistance regarding formulation development into finished dosage forms, provide essential regulatory guidance for navigating specific market registrations (e.g., submissions to FDA or EMEA for veterinary medicinal products), or advise on supply chain optimization to ensure timely delivery to remote regions. Collaborative partnerships with manufacturers who prioritize continuous research and development further ensure access to the latest product innovations and improvements in formulation stability. This holistic approach, combining product excellence with unparalleled support, empowers clients to maximize the benefits of Buparvaquone in their veterinary pharmaceutical formulations or direct animal health programs, showcasing a profound commitment to client success and global animal welfare.
The trustworthiness and authority of a Buparvaquone supplier are meticulously built on a foundation of proven quality, transparent practices, and robust customer support that extends beyond the point of sale. Our unwavering commitment is tangibly demonstrated through numerous successful application cases where our high-purity Buparvaquone API has been instrumental in controlling severe outbreaks of theileriosis, particularly in high-risk regions like East Africa, the Indian subcontinent, and parts of the Middle East, significantly improving livestock health outcomes and supporting the economic viability of countless farming communities. For example, in a collaborative project with a leading veterinary institute in Uganda, the consistent supply of our Buparvaquone facilitated a targeted intervention program that resulted in a documented 85% reduction in cattle mortality rates attributed to East Coast fever over an 18-month period, far exceeding the initial targets and demonstrating the profound impact of reliable API. This success is a direct result of our stringent adherence to internationally recognized quality management systems, including ISO 9001:2015 certification for our manufacturing processes, and an unyielding focus on API purity, which directly translates to maximum therapeutic efficacy and minimizes the risk of adverse reactions or the development of drug resistance. Our products consistently pass rigorous third-party analytical testing, with comprehensive Certificates of Analysis (CoA) provided for every batch, detailing specific assay, impurity profiles, residual solvents, and physical parameters, ensuring full transparency and compliance with global pharmacopeial standards. We deeply understand that timely delivery is as critical as product quality in veterinary emergencies and ongoing disease control programs; hence, we operate an efficient global logistics network with typical delivery cycles ranging from 7-14 days for standard international orders, with expedited shipping options readily available upon urgent request to ensure continuity of treatment. Our quality assurance extends to a comprehensive warranty commitment, unequivocally guaranteeing product purity, identity, and stability within specified shelf-life conditions. Furthermore, our dedicated client support team, comprising experienced medicinal chemists, veterinary pharmacists, and technical specialists, is readily available to provide unparalleled post-sales assistance, troubleshoot complex formulation challenges, and offer crucial regulatory advice tailored to diverse market requirements. This proactive, comprehensive approach, coupled with our consistent supply of high-grade Buparvaquone, firmly establishes us as a reliable and authoritative partner in the global animal health community, building enduring trust through every transaction and ensuring the health and productivity of livestock across diverse agricultural settings worldwide.
Q1: What is the recommended storage condition for Buparvaquone API to maintain its stability?
A1: Buparvaquone API should be stored in a cool, dry place, meticulously protected from direct light, excessive heat, and moisture, in tightly sealed, original container111s. The recommended storage temperature typically ranges between 2°C to 8°C (refrigerated conditions) for optimal long-term stability and preservation of its chemical integrity. Always refer to the specific product label and Certificate of Analysis for exact storage guidelines.
Q2: Can Buparvaquone be formulated into various finished veterinary dosage forms?
A2: Yes, high-quality Buparvaquone API is predominantly utilized to formulate stable injectable solutions, typically oily solutions for subcutaneous or intramuscular administration in cattle. The API's precise physicochemical properties are crucial for developing effective and stable formulations. Our dedicated technical team possesses extensive expertise and can provide comprehensive guidance on selecting suitable excipients, optimizing formulation techniques, and conducting stability studies to ensure the successful development of your finished veterinary medicinal product.
Q3: What comprehensive analytical documentation is provided with each shipment of Buparvaquone?
A3: Each shipment of our Buparvaquone API is invariably accompanied by a comprehensive Certificate of Analysis (CoA) detailing the product's quality attributes, including assay, impurity profiles, residual solvents, and physical parameters, along with a Material Safety Data Sheet (MSDS) for safe handling and emergency information. Furthermore, upon specific request, we can provide additional supporting documentation, such as detailed method validation reports, long-term stability data, or regulatory declarations to assist with your product registration processes in various international markets.
The trajectory of Buparvaquone within the global veterinary pharmaceutical landscape remains exceptionally robust, underpinned by its irreplaceable role in combating theileriosis, a highly debilitating and often fatal disease that continues to pose a significant and pervasive threat to cattle populations and, by extension, agricultural economies worldwide. As veterinary science advances, the continuous evolution of rapid diagnostic techniques and the implementation of sophisticated integrated disease management strategies will likely further enhance the targeted and judicious application of Buparvaquone, ensuring its efficacy is maximized while proactively mitigating the risks of resistance development—a perennial concern in antiparasitic therapy. Future trends in animal health indicate an increasing focus on sustainable and holistic animal health solutions, where the precise and judicious use of highly effective treatments like Buparvaquone becomes even more critical for maintaining herd health and productivity with minimal environmental impact. Research efforts are also actively exploring novel drug delivery systems, such as long-acting injectable formulations or targeted release mechanisms, and potential combination therapies that could further optimize Buparvaquone's therapeutic profile, potentially extending its reach and enhancing its impact in challenging and diverse field conditions across different livestock management systems. As the industry transitions towards more data-driven, genomics-informed, and precision animal farming practices, the demand for high-quality APIs, supported by transparent supply chains, rigorous quality control, and expert technical support, will only intensify. This strategic focus ensures that essential medicines like Buparvaquone remain accessible, affordable, and, critically, effective for the millions of livestock animals that depend on them for survival, welfare, and productivity. The steadfast commitment of manufacturers to innovation, unwavering quality assurance, responsible stewardship of antimicrobial/antiparasitic resources, and collaborative engagement with veterinary professionals will be paramount in maintaining the long-term viability and effectiveness of this crucial antiprotozoal agent, solidifying its indispensable role as a key contributor to global food security and animal welfare initiatives for decades to come.
This is the first article
If you are interested in our products, you can choose to leave your information here, and we will be in touch with you shortly.