
The global pharmaceutical landscape is constantly evolving, driven by the emergence of new diseases, drug resistance, and a heightened focus on public health. In this dynamic environment, the demand for high-quality Active Pharmaceutical Ingredients (APIs) remains critical. Among these, Atovaquone stands out as a crucial compound with significant implications for global health, particularly in the treatment and prevention of parasitic infections. Recent industry trends indicate a growing emphasis on vertically integrated supply chains, ensuring consistent quality, robust regulatory compliance, and sustainable manufacturing practices for essential APIs like Atovaquone. The market is increasingly demanding APIs that not only meet stringent purity and potency requirements but also offer excellent bioavailability and predictable pharmacokinetic profiles. Furthermore, the push for eco-friendly synthesis methods and reduced waste generation is shaping the future of pharmaceutical manufacturing. This compound’s efficacy against specific pathogens positions it as a cornerstone in medical protocols, driving sustained demand and necessitating reliable, high-volume production capabilities. As global health organizations and governments prioritize infectious disease control, the strategic importance of this API continues to escalate, underscoring the need for advanced manufacturing expertise and a deep understanding of its chemical properties and biological activity. This includes meticulous control over polymorphism and particle size distribution to ensure optimal drug formulation and therapeutic outcomes.
Beyond its immediate medical applications, the research and development pipeline for related compounds and new delivery systems for Atovaquone is robust, indicating its long-term relevance. Pharmaceutical companies are exploring combination therapies to enhance efficacy and mitigate resistance, further solidifying its role in therapeutic regimens. The demand for API manufacturers who can provide comprehensive data packages, including impurity profiles, stability data, and validation reports, is at an all-time high. This reflects the industry's commitment to patient safety and drug integrity. Suppliers who can demonstrate a proven track record of adherence to Good Manufacturing Practices (GMP) and international regulatory standards, such as those set by the FDA, EMA, and WHO, are becoming preferred partners. The current trends also highlight the critical role of intellectual property management and secure supply chains to prevent counterfeiting and ensure the availability of authentic, life-saving medications. The specialized expertise required to produce such a complex API reliably and at scale distinguishes leading manufacturers in the highly competitive B2B pharmaceutical market, making strategic partnerships essential for ensuring global drug accessibility.
Understanding the precise technical specifications of Atovaquone is paramount for pharmaceutical formulators and researchers. As a highly specialized API, its efficacy and safety are directly linked to its chemical purity, physical properties, and crystallographic characteristics. Manufacturers must adhere to strict quality control parameters to ensure batch-to-batch consistency and compliance with pharmacopeial standards such as the United States Pharmacopeia (USP), European Pharmacopoeia (EP), and British Pharmacopoeia (BP). Key parameters include molecular formula, molecular weight, CAS number, appearance, purity profile (typically determined by High-Performance Liquid Chromatography, HPLC), melting point, water content (Karl Fischer), heavy metal content, residual solvents, and specific optical rotation, if applicable. These parameters collectively define the chemical identity and purity, which are critical for its integration into various dosage forms and for maintaining its therapeutic activity. Variations in these specifications can profoundly impact drug stability, dissolution rates, and ultimately, patient outcomes. Therefore, selecting a supplier capable of consistently delivering an API that meets or exceeds these rigorous specifications is a non-negotiable aspect of pharmaceutical development and manufacturing.
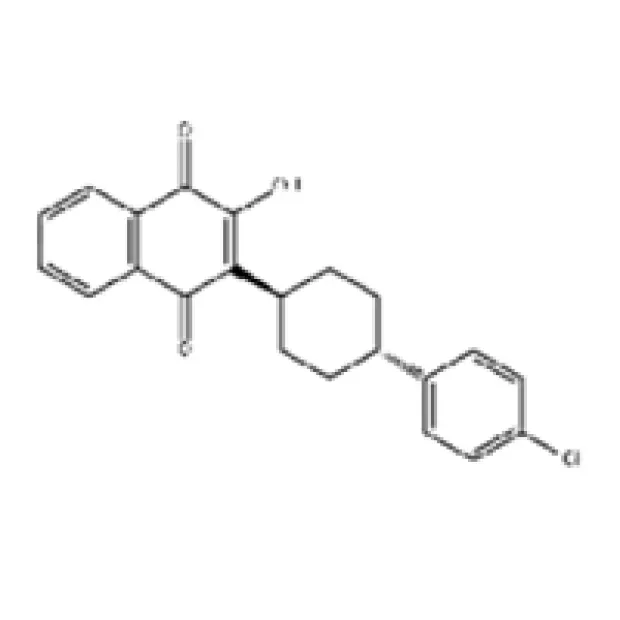
| Parameter | Specification | Method/Standard |
|---|---|---|
| Molecular Formula | C22H18O3 | Empirical Formula Analysis |
| Molecular Weight | 366.38 g/mol | Calculated |
| CAS Number | 95233-18-4 | Registry Identification |
| Appearance | Yellow Crystalline Powder | Visual Inspection |
| Purity (HPLC) | ≥ 99.0% | USP/EP Monograph |
| Melting Point | 210-215°C | Capillary Method |
| Solubility | Practically insoluble in water; soluble in DMSO, DMF | Solubility Test |
| Loss on Drying | ≤ 0.5% | Karl Fischer Titration |
These detailed specifications are crucial for ensuring the stability and bioavailability of the final drug product. For instance, the crystalline form of Atovaquone can significantly influence its dissolution rate and absorption in the body, necessitating precise control over the crystallization process during manufacturing. Strict adherence to these specifications is not just about compliance; it's about delivering therapeutic efficacy and ensuring patient safety. Our commitment to rigorous quality control protocols ensures that every batch of Atovaquone meets these exacting standards, providing a reliable and consistent API for pharmaceutical formulations globally.
The manufacturing of Atovaquone is a complex, multi-step chemical synthesis process that demands meticulous control over reaction conditions, raw material quality, and intermediate purification. Our robust production methodology is designed to yield a high-purity API suitable for pharmaceutical applications, adhering strictly to current Good Manufacturing Practices (cGMP) guidelines. The process typically commences with the careful selection and qualification of high-grade raw materials, each undergoing rigorous inspection to ensure their identity, purity, and freedom from contaminants. Subsequent stages involve a series of precisely controlled chemical reactions, often including cyclization and condensation steps, executed under optimized temperature, pressure, and solvent conditions. Each reaction step is monitored in real-time using advanced analytical techniques like Gas Chromatography (GC) and Liquid Chromatography-Mass Spectrometry (LC-MS) to track reaction progress, minimize side-product formation, and maximize yield. The choice of catalysts and reagents is critical, as it directly impacts reaction efficiency, product purity, and environmental footprint. Our processes are continuously refined through process analytical technology (PAT) to ensure robust and reproducible synthesis.
Following the synthesis, the crude Atovaquone undergoes extensive purification to remove impurities, unreacted starting materials, and by-products. This purification typically involves multiple recrystallization steps, often employing different solvent systems, filtration, and specialized chromatography techniques to achieve the desired purity profile. The final product is then subjected to a carefully controlled drying process to achieve optimal moisture content, which is crucial for stability and shelf life. Throughout the entire manufacturing chain, from raw material receipt to final packaging, stringent in-process controls and quality assurance checks are implemented. This includes regular sampling and testing against predefined specifications using a suite of analytical methods, such as Fourier-Transform Infrared (FTIR) spectroscopy for structural confirmation, X-ray diffraction (XRD) for polymorph identification, and Inductively Coupled Plasma – Mass Spectrometry (ICP-MS) for heavy metal analysis. Our facilities are ISO 9001 certified, demonstrating our commitment to a comprehensive quality management system that spans every aspect of production. The use of advanced engineering controls and robust validation protocols ensures that our Atovaquone consistently meets the highest international standards, providing a reliable API for critical medical treatments.
Atovaquone is a highly effective naphthoquinone derivative with potent antiprotozoal activity, making it an indispensable API in various critical medical applications. Its primary clinical utility lies in the treatment and prophylaxis of malaria, particularly against drug-resistant strains of Plasmodium falciparum, often used in combination with Proguanil. This combination therapy leverages synergistic mechanisms to effectively clear parasitic infections and prevent their recurrence. Furthermore, it is a first-line treatment for Pneumocystis jirovecii pneumonia (PCP), a severe opportunistic infection common in immunocompromised individuals, such as those with HIV/AIDS or undergoing immunosuppressive therapy. Its efficacy in these life-threatening conditions underscores its significant role in public health. Beyond these established uses, Atovaquone is also being explored for its potential in treating other parasitic diseases, reflecting its broad-spectrum antiparasitic properties. The ability to prevent and treat these severe infections positions it as a vital compound in global health initiatives, offering a robust solution where conventional treatments may be less effective or prone to resistance. Its technical advantages stem from its unique mechanism of action, selectively inhibiting the parasite's mitochondrial electron transport, thus disrupting essential metabolic processes without significant harm to the host.
The technical advantages of Atovaquone extend beyond its direct antiparasitic action. Its relatively favorable safety profile, compared to some older antimalarials, makes it a preferred choice for a wider range of patients, including pediatric populations and those with specific co-morbidities. Its oral bioavailability, while influenced by food intake, allows for convenient administration, enhancing patient adherence to treatment regimens. For manufacturers, the key technical advantage lies in the ability to produce a consistently pure and stable API that meets the stringent requirements for formulation into tablets or suspensions. Our refined manufacturing processes ensure high purity, reducing the risk of adverse reactions stemming from impurities and contributing to the overall stability and shelf life of the final drug product. This high-quality API ensures optimal drug performance in diverse environmental conditions, critical for global distribution. Moreover, the compound's stability under various storage conditions contributes to its longer shelf life, reducing waste and ensuring drug availability. In terms of formulation, its chemical structure allows for effective incorporation into various drug delivery systems, offering flexibility for pharmaceutical companies. The consistent performance of Atovaquone in challenging clinical environments highlights its robust technical design and the precision required in its synthesis and quality assurance.
In the highly competitive pharmaceutical API market, the ability to provide customized solutions for Atovaquone sets a manufacturer apart. Recognizing that different drug formulations and applications may require specific characteristics, we offer tailored manufacturing services to meet unique client requirements. This includes the ability to control particle size distribution through micronization or milling techniques, which is critical for enhancing dissolution rates and bioavailability in the final dosage form. We can also provide different crystalline forms or polymorphs of Atovaquone, as specific polymorphic forms can have distinct solubility and stability profiles relevant for particular drug products. Our expertise extends to packaging options, offering custom container111 sizes, specialized inert atmosphere packaging for enhanced stability, or bulk quantities to suit varied production scales. Our technical team works closely with clients, from research and development phases through commercial production, to understand their specific needs and provide solutions that optimize their drug development process. This collaborative approach ensures that the API supplied seamlessly integrates into the client’s manufacturing workflow, minimizing potential issues and accelerating time to market. We understand that success in pharmaceutical manufacturing relies on precise control and adaptability, hence our dedication to providing bespoke solutions for our partners.
Our commitment to excellence extends beyond customization to encompass robust quality control and an unparalleled service record. While direct manufacturer comparison by name is not feasible, the key differentiating factors to consider in any API supplier for Atovaquone include stringent adherence to global regulatory standards (e.g., US FDA, EMA, WHO), a comprehensive quality management system (e.g., ISO 9001 certified), proven analytical capabilities, and a transparent supply chain. Our facilities boast state-of-the-art analytical laboratories equipped with advanced instrumentation for purity profiling, impurity identification, and stability testing, providing comprehensive Certificates of Analysis (CoA) for every batch. With over a decade of experience in API synthesis and a dedicated team of chemists and regulatory experts, we consistently deliver high-quality Atovaquone. Our established track record of successful regulatory audits and consistent supply to major pharmaceutical companies globally underscores our reliability and expertise. We prioritize long-term partnerships built on trust, transparency, and the consistent delivery of superior product quality and technical support. This holistic approach ensures that our clients receive not just an API, but a comprehensive solution backed by extensive experience and unwavering commitment to pharmaceutical excellence.
Our Atovaquone API has been successfully utilized by numerous pharmaceutical companies globally in the formulation of critical antimalarial and antipneumocystis drugs. In one notable case, a leading generics manufacturer in Southeast Asia leveraged our high-purity Atovaquone to develop a cost-effective generic version of a combination antimalarial drug. Our consistent product quality, coupled with comprehensive regulatory documentation, facilitated their swift approval by local health authorities, enabling them to address a significant public health need in regions endemic with drug-resistant malaria. Another instance involved a European pharmaceutical firm that required a specific particle size distribution of Atovaquone for an innovative pediatric suspension formulation. Through our custom milling capabilities and dedicated technical support, we provided an API that met their exacting specifications, resulting in a stable and bioavailable product that significantly improved medication adherence in young patients. These examples highlight not only the versatility of our product but also our capability to partner with clients to overcome complex formulation challenges, ultimately benefiting patients worldwide. Our commitment to supporting these critical applications underscores our role as a reliable supplier in the global pharmaceutical supply chain.
Our commitment to quality extends to a comprehensive product warranty, ensuring that every batch of Atovaquone meets the agreed-upon specifications and regulatory standards. Our delivery cycle is optimized for efficiency and reliability, with robust supply chain management to ensure timely and secure global shipments. We provide extensive customer support, including technical assistance, regulatory documentation, and post-sales service, ensuring a seamless experience for our partners. This holistic approach builds trust and reinforces our position as a leading supplier of high-quality APIs.
This is the first article
Urea Formula 98% Chromatographic Purity
If you are interested in our products, you can choose to leave your information here, and we will be in touch with you shortly.